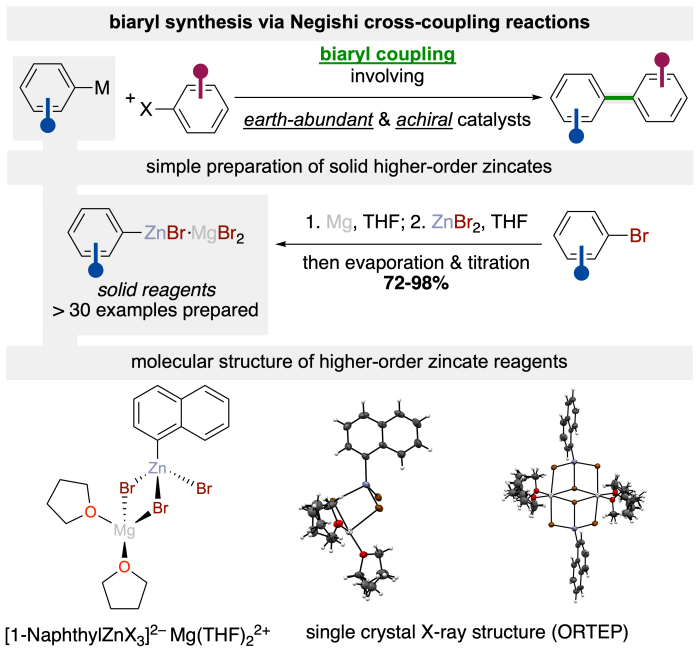
Coupling reactions employing higher-order zincates
We are currently developing different (asymmetric) approaches towards the synthesis of biaryls via Ni/N,N-ligand catalyzed Negishi cross-coupling reaction.
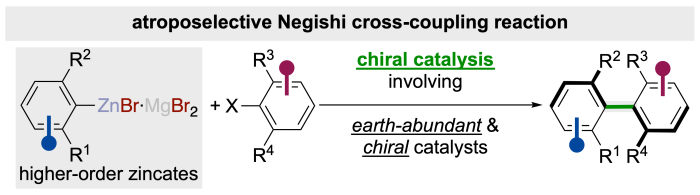
In the recent past, this approach has already been adapted in the development of an atroposelective variant of the former Negishi cross-coupling reaction. Beside its discovery and development, we are currently still working on examination of the underlying reaction mechanism of this particular cross-coupling reaction, since it might also important to other earth-abundant transition-metal catalyzed cross-coupling reactions especially emplyoing zinc reagents.
Although the asymmetric cross-coupling reaction towards atropoisomers controlled via a chiral catalyst is undoubtedly the most efficient and potent method for their preparation, it is often accompanied by the issue of the often complicated preparation of sensitive chiral catalyst and the limitations regarding inaccessible products. Therefore, we believe that utilizing abnormal easily synthesizable chiral directing group in the hailde substrate is useful alternative. However, the chiral directing group must be easily transformed into various functional groups in short sequences under retention of the axial stereo information.

We are also interested in applying our novel, highly transmetallating active higher-order zincates in oxidative homo-coupling reactions, especially in an atroposelective fashion employing (chiral) earth-abundant catalysts. Employing sustainable oxidizing reagents, peferably oxygen, we might develop an useful alternative to recent developments in (atroposelective) reductive homo-coupling reactions.

In this context, we are – of course – also interested in the application of this methodolgies in the total synthesis of atropoisomeric natural products and potentially interesting biaryls in the future.